Impact of Heat Treatment on Mechanical Properties and Thermal Conductivity of ZL102 Alloy
The impact of heat treatment on the mechanical properties and thermal conductivity of die-cast ZL102 aluminum alloy was investigated through microstructure characterization and performance testing. The room-temperature microstructure of die-cast ZL102 aluminum alloy comprises primary α (Al), aluminum-silicon eutectic structures, primary silicon, and trace intermetallic compounds. Solution treatment causes the silicon phase to dissolve and spheroidize. Aging treatment results in the precipitation of fine, point-like second-phase particles on the α (Al) matrix, enhancing the sphericity of the silicon phase at grain boundaries. Among the three heat treatment conditions, the alloy demonstrates the highest mechanical properties after solution treatment, though its thermal conductivity is the lowest. Overall, aging treatment provides a balance between mechanical properties and thermal conductivity. Under these conditions, the alloy achieves a tensile strength of 212 MPa, an elongation of 3.9%, and a room-temperature thermal conductivity of 142.7 W/(m·K).
The Role of Aluminum Alloys in Heat Dissipation for 5G Communication Devices
With the advancement of 5G communication technology, electronic devices and systems are becoming increasingly integrated, resulting in higher heat generation per unit volume. This creates a demand for materials and structures with superior thermal conductivity to maintain device performance and prolong service life. For example, 5G communication filters, which are characterized by high power and integration, often require heat dissipation solutions. To address this, filter enclosures are commonly designed with irregular, thin-walled heat sinks. Die-casting is an efficient and cost-effective manufacturing method for mass-producing such structural enclosures.
Aluminum, with a density about one-third that of steel or iron, offers significant weight reduction potential. In recent years, aluminum has found widespread use in industries such as automotive, communications, and aerospace. Pure aluminum exhibits excellent thermal conductivity, approximately 237 W/(m·K) at room temperature. However, its relatively low strength necessitates the addition of alloying elements to enhance its mechanical properties, which may, in turn, reduce its thermal conductivity. Typically, alloying elements improve the performance of aluminum alloys through mechanisms such as solid solution strengthening, intermediate phases, and precipitation-strengthening phases. However, these processes introduce crystal defects, such as vacancies and dislocations, and cause lattice distortion due to the precipitation phases. These defects increase the likelihood of free electron scattering, reducing the number of electrons available for heat conduction and subsequently lowering the alloy's thermal conductivity.
Extensive studies have been conducted to optimize the balance between the mechanical and thermal conductivity of aluminum alloys. Cheng Wen investigated the effects of 22 alloying elements on the electrical and thermal conductivity of industrial pure aluminum, noting that the impact varied significantly across different elements. Transition elements, such as Mn and Cr, were found to reduce both electrical and thermal conductivity, whereas elements like Zn, Sr, and rare earth modifiers had comparatively minor effects.
Linjun Li examined the influence of magnesium-silicon ratios on the thermal conductivity of aluminum alloy 6063, determining that an optimal ratio of 1.5 yielded the best thermal performance. Lumley et al. explored how alloy composition and heat treatment conditions affect the thermal conductivity of Al-Si-Cu aluminum alloy die castings, revealing that heat treatment could increase thermal conductivity by more than 60% for specific alloy compositions. Kim et al. measured the thermal diffusivity of Al-1Si and Al-9Si alloys under varying heat treatment conditions, analyzing the relationship between diffusivity and the silicon phase, whether in solid solution or precipitated form. They concluded that the re-precipitation of dissolved silicon in solution-treated samples significantly enhanced the alloy’s thermal diffusivity. Choi et al. investigated the effects of mold temperature on the thermal and mechanical properties of aluminum alloys, finding that higher mold temperatures slowed the solidification rate, increased silicon particle size, and improved thermal properties. After aging treatment, the mechanical strength of alloys produced at different mold temperatures became comparable.
This study focuses on the die-casting of aluminum alloy ZL102 for use in 5G communication filter housings. The flash method was employed to measure the thermal conductivity of the alloy at various temperatures. Heat treatment was applied to modify the alloy’s microstructure and evaluate its effects on both mechanical and thermal properties, with the aim of providing valuable insights to improve the performance of aluminum alloy die-castings in production.
1. Experimental Process
1.1 Sample Preparation
The test specimen is a die-cast 5G communication filter housing, shown in Figure 1a. It weighs approximately 5.4 kg and has dimensions of 539 mm × 410 mm × 45 mm. The die-cast product features a complex structure, with the main wall thickness being about 2 mm and a maximum thickness of 6 mm at the installation lug and other areas. Microstructure characterization, mechanical testing, and thermal conductivity testing were performed at the sampling location indicated in Figure 1b, which corresponds to the main body of the die-cast product, ensuring representativeness and convenience for sampling.
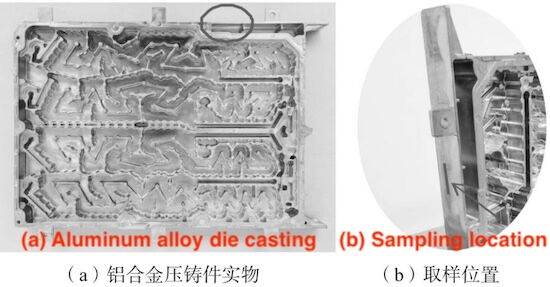
Figure 1 Photograph of the die-casting and sampling location
The chemical composition of aluminum alloy ZL102, determined using an X-ray fluorescence spectrometer (XRF), is shown in Table 1. Tensile specimens for mechanical testing were prepared by wire cutting, with dimensions in compliance with the national standard GB/T 228.1-2010. Circular specimens, with a diameter of 4 mm and a thickness of 1 mm, were also wire-cut for thermal conductivity testing. Heat treatment was applied to the specimens in three control groups: the first group was die-cast without heat treatment, the second group underwent solution treatment at 500°C for 4 hours, and the third group underwent aging treatment at 200°C for 3 hours, following the second group.
1.2 Test Method
The metallographic samples were sequentially ground with sandpapers of 240#, 600#, 1200#, 1500#, and 2000# grit, then polished using a diamond polishing agent. The samples were etched with a Keller reagent (95% H2O, 2.5% HNO3, 1.5% HCl, and 1% HF) for 10–20 seconds. The microstructure was analyzed using a Leica DM2700M optical microscope (OM) and a JEOL 6301F scanning electron microscope (SEM). Room-temperature tensile testing was conducted with an Instron 5967 electronic universal testing machine at a tensile rate of 2 mm/min. The mechanical properties data represent the average of five valid samples.
Due to the complexity of direct thermal conductivity measurement, the thermal conductivity of the alloy was calculated based on the laws of heat conduction using equation (1), incorporating measurements of density, specific heat capacity, and thermal diffusion coefficient:
λ=αρCρ(1)
Where:
- λ is the thermal conductivity (W/(m·K)),
- α is the thermal diffusion coefficient (mm²/s),
- ρ is the density (g/cm³),
- Cρ is the specific heat capacity (J/(g·K)).
The thermal diffusion coefficient was measured using an LFA457 laser thermal conductivity meter, the specific heat capacity was measured with a TA-DSC2500 differential scanning calorimeter, and the density was determined using the Archimedes displacement method.
2. Die-Casting Structure of Aluminum Alloy ZL102
The filter housing is die-cast from aluminum alloy ZL102, which contains 12.8% silicon by mass (all subsequent references are by mass), close to the eutectic composition of the aluminum-silicon binary alloy (12.6% Si at the eutectic composition point in the Al-Si phase diagram). During solidification, the eutectic reaction L → α(Al) + β(Si) predominantly occurs, forming a substantial amount of α(Al) + Si eutectic structure. However, due to the high cooling rate during die-casting and the solidification process deviating from the equilibrium phase diagram, the phases in the die-cast structure of aluminum alloy ZL102 at room temperature include primary α(Al), aluminum-silicon eutectic structure, primary silicon, and small quantities of intermetallic compounds.
Figure 2 illustrates the surface and core microstructures of the die-cast product, obtained via optical microscopy (OM). The gray-white matrix consists primarily of α(Al), with a substantial amount of gray-black aluminum-silicon eutectic structure and needle- or lath-shaped primary silicon distributed within the matrix. Comparison of Figure 2a and Figure 2b reveals a finer and more uniform surface structure. This is due to the higher cooling rate and significant supercooling that the die-cast surface experiences as it directly contacts the mold wall during formation, resulting in finer grains. Additionally, a small amount of large, gray, polygonal structures is observed in the core structure of the die-cast product, as shown in Figure 2b. Given the slightly higher iron content in the alloy, these structures are preliminarily identified as iron-containing phases. X-ray diffraction (XRD) analysis was conducted to determine the phase composition of the alloy, with a scanning range of 10° to 90° and a scanning speed of 4°/min. The results, processed using MDI Jade 6 software, are shown in Figure 3. The XRD analysis confirmed the presence of only α(Al) and Si phases, with no iron-containing intermetallic compounds detected. This may be attributed to the low content of intermetallic compounds in the alloy, which prevented their detection in the XRD analysis.
Table 1: Chemical Composition of Aluminum Alloy ZL102 (wB/%)
|
Element |
Si |
Mg |
Fe |
Mn |
Cu |
Zn |
Ti |
Al |
|
Content |
12.8 |
0.019 |
0.915 |
0.184 |
0.224 |
0.127 |
0.001 |
Balance |
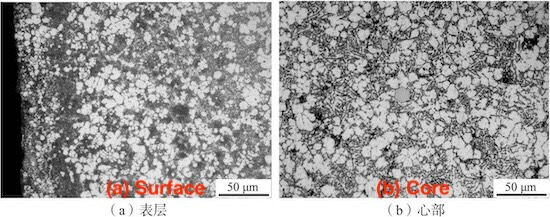
Figure 2: Microstructure of Die-Cast Aluminum Alloy ZL102
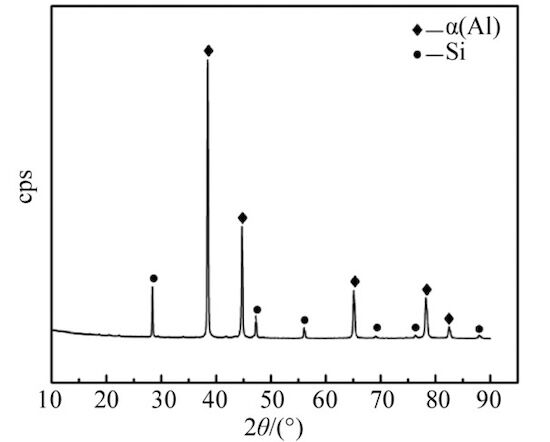
Figure 3: XRD Spectrum of Die-Cast Aluminum Alloy ZL102
The polygonal phase, previously mentioned, was further analyzed using scanning electron microscopy (SEM), as shown in Figure 4. Based on the energy dispersive spectrometer (EDS) results presented in Table 2, this phase is identified as a complex AlSiFeMn quaternary intermetallic compound. Studies, including those by Yuan and Wang, confirm that this phase corresponds to α-Al15(Mn, Fe)3Si2, which forms through the addition of Mn to the β-Al5FeSi phase. During the formation of β-Al5FeSi, Mn atoms partially replace Fe atoms, facilitating the development of the AlSiFeMn quaternary composite phase.
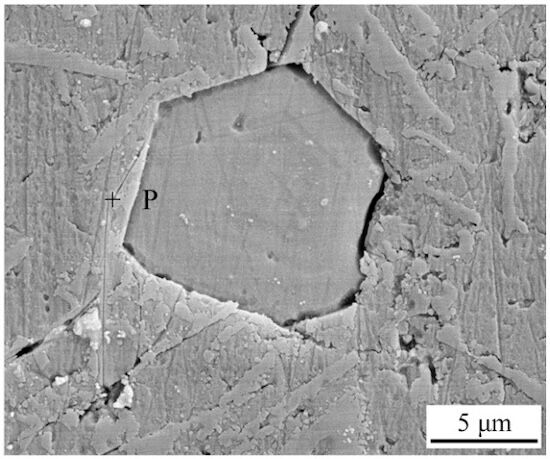
Figure 4 SEM image of polygonal phase
Table 2 EDS analysis results of point P in Figure 4 %
|
Element |
Mass Fraction (%) |
Atomic Fraction (%) |
|
Al |
54.63 |
65.72 |
|
Si |
13.38 |
15.47 |
|
Fe |
21.79 |
12.67 |
|
Mn |
6.52 |
3.85 |
|
Cr |
3.67 |
2.29 |
2. Effect of Heat Treatment on the Die-Casting Structure
Figure 5 presents a comparative analysis of the die-casting structure of aluminum alloy ZL102 before and after heat treatment. The primary α (Al) phase shows minimal changes, with no significant variations in dendrite orientation before or after heat treatment. However, the morphology and distribution of the silicon phase undergo significant alterations. In the as-cast structure, the silicon phase appears in a needle-like or lath-like form, disrupting the α (Al) matrix. After solution treatment, the silicon phase dissolves and transforms into spherical particles, reducing the aspect ratio and increasing sphericity. During aging treatment, silicon previously dissolved in the α (Al) matrix precipitates, forming fine, point-like structures on the matrix. Additionally, the silicon phase at the grain boundaries becomes more rounded, further improving sphericity. The morphology of the polygonal AlSiFeMn quaternary intermetallic compound in the die-casting structure remains unchanged during solution and aging treatments. This suggests that the solution treatment temperature in this study was insufficient to dissolve the compound into the α (Al) matrix. Figure 5 also shows a noticeable distribution of pores within the die-casting structure. The number of pores increases after solution treatment, but no further expansion occurs following aging treatment.

Figure 5 Comparative analysis of die-cast, solution, and aging structures
4. Effect of Heat Treatment on Mechanical Properties and Thermal Conductivity
Figure 6 illustrates the mechanical properties of die-cast aluminum alloy ZL102 after various heat treatment processes. The solution-treated sample demonstrates superior strength and elongation compared to both the die-cast and aged samples. The tensile strength and elongation of the solution-treated sample are 222.8 MPa and 6.1%, respectively. Compared to the die-cast state, the solution-treated sample exhibits a 9.2% increase in tensile strength and a 205% improvement in elongation. The mechanical properties of the aged sample fall between those of the die-cast and solution-treated samples.
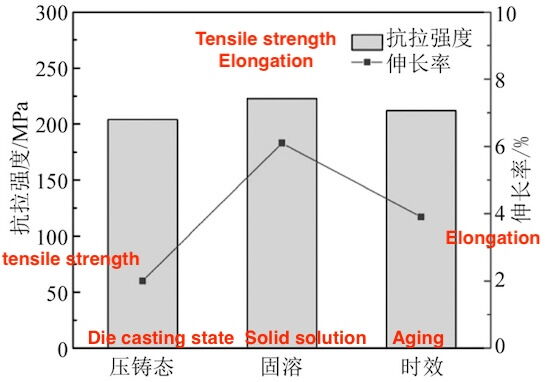
Figure 6 Effect of heat treatment on the mechanical properties of die-cast aluminum alloy ZL102
While some studies suggest that conventional die-castings are unsuitable for heat treatment due to porosity, and that aluminum alloy ZL102 typically does not benefit from heat treatment, the melting and spheroidization of the silicon phase during solution treatment significantly improve its interaction with the α (Al) matrix. This process reduces stress concentrations under loading, enhancing the alloy’s strength and elongation. After aging treatment, the mechanical properties of the alloy show a slight decline compared to the solution-treated state. This decrease is attributed to the aggregation and coarsening of the silicon phase at the α (Al) grain boundaries and within the solid solution, which deteriorates the alloy's mechanical properties. Additionally, no strengthening phases form during the aging process.
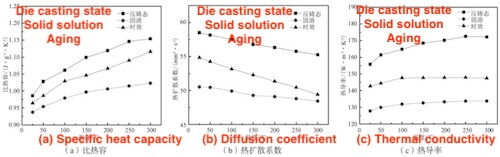
Figure 7 Effects of Temperature and Heat Treatment Process on Thermal Conductivity of Die Casting Aluminum Alloy ZL102
Figure 7 illustrates the experimental results showing how varying temperatures and heat treatment processes influence the thermal conductivity of die-cast aluminum alloy ZL102. The alloy's thermal conductivity increases with temperature in its die-cast, solution-treated, and aged states. Initially, the increase is rapid, then slows gradually between room temperature and 300°C. This pattern is attributed to the combined electronic and phonon contributions to the alloy's thermal conductivity. In metals, thermal conductivity arises from both electronic and phonon effects, while thermal resistance is similarly divided into electronic and phonon components. Electronic thermal resistance occurs due to electron scattering by various media, such as electron-phonon and electron-defect interactions. Phonon thermal resistance is influenced by lattice vibrations and interactions between phonons and defects.
At lower temperatures, atomic vibrations in the lattice are minimized, leading to negligible phonon contribution to thermal conductivity. In this range, electronic thermal conductivity predominates, and the mean free path of electrons remains largely unchanged. As a result, thermal conductivity is mainly determined by specific heat, which has a first-order relationship with temperature, causing a rapid initial increase in conductivity under all three heat treatment conditions. As temperature continues to rise, atomic vibrations intensify, leading to increased electron scattering by phonons and a reduction in the mean free path of electrons. This results in a gradual slowdown in the rate of increase in thermal conductivity.
Figure 7 shows that, among the three heat treatment conditions, the die-cast alloy exhibits the highest thermal conductivity across all temperatures. In contrast, both solution and aging treatments lead to a reduction in the thermal conductivity of aluminum alloy ZL102. This decrease is primarily attributed to the high concentration of solute atoms introduced during solution treatment, which causes lattice distortion and increases crystal defects. These defects enhance electron scattering, reduce the mean free path of electrons, and significantly lower the alloy's thermal conductivity. Following aging treatment, the alloy's thermal conductivity partially recovers compared to its solution-treated state. This recovery occurs because aging induces the precipitation of a secondary phase, which decreases the solid solubility of the alloy and alleviates lattice distortion. However, the thermal conductivity of the aged alloy remains lower than that of the die-cast alloy. This reduction is due to the formation of additional phase interfaces from secondary phase precipitation, which increases electron scattering and further reduces thermal conductivity.
Overall, the effect of alloying elements on the thermal conductivity of aluminum alloy ZL102 is more detrimental in the solid solution state than in the form of precipitates. Additionally, grain growth and pore formation during heat treatment also impact thermal conductivity. Studies indicate that grain growth reduces crystal interfaces, which slightly enhances thermal conductivity, while pore formation significantly decreases it.
Conclusion
- At room temperature, the die-cast structure of aluminum alloy ZL102 consists of primary α (Al), an aluminum-silicon eutectic structure, primary silicon, and small amounts of intermetallic compounds. The polygonal phase is identified as the AlSiFeMn quaternary composite phase.
- Solution treatment causes the silicon phase in the die-cast structure to dissolve and spheroidize, while aging treatment leads to the precipitation of fine, point-like second-phase particles on the α (Al) matrix. This process also enhances the sphericity of the silicon phase at the grain boundaries.
- Among the three heat treatment states, the solution-treated alloy exhibits the highest mechanical properties, with a tensile strength of 222.8 MPa and elongation of 6.1%. These values represent increases of 9.2% and 205%, respectively, compared to the die-cast state. However, the thermal conductivity of the solution-treated alloy is the lowest, decreasing from 155.8 W/(m·K) in the die-cast state to 127.8 W/(m·K) at room temperature.
In summary, aging treatment provides a balance between mechanical properties and thermal conductivity. Under these conditions, the alloy achieves a tensile strength of 212 MPa, elongation of 3.9%, and a room temperature thermal conductivity of 142.7 W/(m·K).
Related News
- Impact of Heat Treatment on Mechanical Properties and Thermal Conductivity of ZL102 Alloy
- Impact of T6 Heat Treatment on ADC12 Aluminum Alloy Properties
- Enhancing the Mechanical Properties of ADC12 Aluminum Alloy via T6 Heat Treatment
- Die-Casting Process Design of Valve Bodies for Automobile Oil Cylinder Parts
- Temperature Field Simulation & Optimization of Automotive Housing Die Castings
- Research Status of High-Impact Aluminum Alloys Domestically and Internationally
- Die-cast Aluminum Castings for High-speed Rail Rocker Arm Shells
- Aluminum Alloys for Automobile Body Panels
- Analysis and Measures of Internal Shrinkage Cavities in Aluminum Die Castings
- Advantages of Aluminum Alloys in Lightweight Automobiles


